Tackling Nuclear Waste Challenges One Atom at a Time
Nuclear energy is having a bit of a moment, with recent interest from big tech companies like Microsoft, Google, Amazon and Meta, which are pursuing nuclear energy as a sustainable and reliable source of power for their growing data centers.
While nuclear energy is a clean energy source, it does produce radioactive waste that stays toxic for tens of thousands of years and needs to be properly disposed of. Disposal typically involves placing nuclear waste in a corrosion-resistant container that is then placed in a repository underground.
Over time, nuclear waste can become unsafe, reacting with water vapors and forming a flammable hydrogen gas.

Rajat Goel, a Ph.D. student in chemical engineering at the University of California, Davis, has embarked on an epic computational quest, seeking to understand how the reaction starts.
“I’m focusing on understanding the corrosion mechanism and how the hydrogen penetrates the uranium and reacts with it,” he said. “If we can understand the mechanism, we can then develop mitigation strategies, be it a coating or other possible solutions.”
With funding from and in collaboration with Lawrence Livermore National Lab, or LLNL, Goel used supercomputing tools to investigate the reaction between hydrogen and uranium oxide at the atomic level — a necessary step to making nuclear energy safer.
Powering Discovery with Computational Chemistry
In the fall of 2022, Goel embarked on his Ph.D. thesis: investigating certain grain boundaries, or structural imperfections, in uranium oxide to figure out which ones are more reactive to hydrogen. Instead of physical experiments, which are costly and dangerous, Goel used a powerful quantum chemistry method called Density Functional Theory, or DFT.
DFT promises very high accuracy, but, according to Goel, it has its drawbacks.
“It’s extremely slow,” said Goel. “We were dealing with 400 atoms of uranium and oxygen, which is a lot of atoms and electrons, and we wanted to do a thorough study of the entire breadth of possible hydrogen interactions with this metal. But we didn’t want to lose accuracy.”
Under the mentorship of Associate Professor of Chemical Engineering Ambar Kulkarni and Nir Goldman, an adjunct faculty member in chemical engineering and scientist at LLNL, Goel used the High Performance Computing Core Facility at UC Davis for about a year and then the supercomputers at LLNL to perform his computations.
He realized he needed more computing power. Goel then caught wind of a student allocation program from the National Renewable Energy Laboratory, or NREL, which was looking for students to try out a new supercomputer called Kestrel.
Goel was granted 16,000 GPRs, or graphic processing unit resource hours, for his analysis. That’s enough processing power to stream Netflix in HD nonstop for nearly two years.
Where Hydrogen Meets Uranium
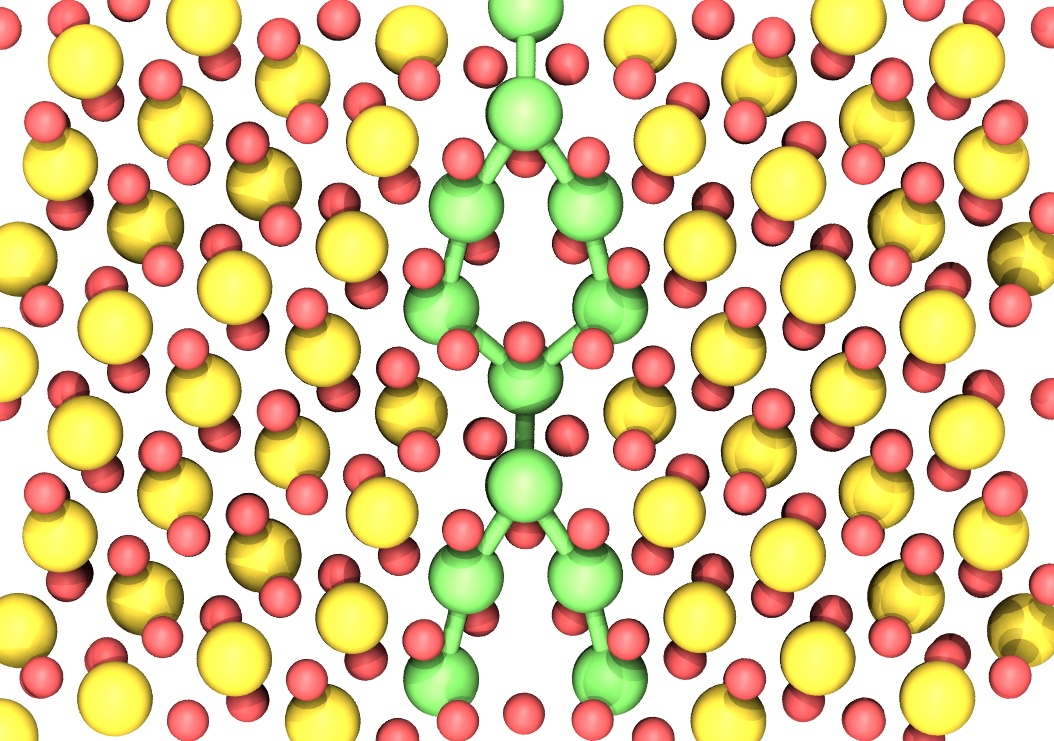
Using DFT and Kestrel, Goel was able to study three common types of grain boundaries, simulate over 100 possible spots where hydrogen might stick, calculate how strong the attraction, or binding energy, is at each of those spots, and analyze the results to see what kinds of atomic environments made the hydrogen bind better.
The researchers found that the geometry and electronic structure, or how the electrons are arranged around the atoms, control how strongly the hydrogen binds. Hydrogen prefers the environment of grain boundaries that have a loose, open shape. If the electrons around uranium atoms are more available, they can help stabilize the hydrogen atom when it binds.
They also discovered that hydrogen attaches to uranium atoms, not oxygen and that when the hydrogen binds, electrons move around from the uranium to the hydrogen, which helps stabilize the bond. The method and results are published in the Journal of Physical Chemistry.
Toward a Safer Nuclear Future
As Goel continues this line of research, he aims to push computing to its potential, using new and established tools to build a foundation of understanding of nuclear waste and develop strategies to make nuclear energy safer and more reliable.
“I’m trying to find out how to use computers to study a real-world problem,” he said. “Uranium is a very complex material because of its electronic configuration, but computational studies have come a long way and are mature enough to simulate these materials.”